Determination of moisture is one of the most important experiment in Food Science & Technology, also in food industry. In this blog, you will learn about the importance, methods & various facts about the analysis of moisture in any food sample.
Why moisture content is estimated?
- To determine Microbial stability of food by knowing moisture % & water activity (aw)
- In order to maintained food quality & safety
Industrial applications of determination moisture content :
- Moisture content in food can have a significant impact on factors such as the product’s taste, texture, appearance, shape & weight
- For Legal and labeling requirements
- To study Shelf life
- To get idea about Food handling & processing operations
Methods determine Moisture content in food
1. AIR-OVEN METHOD or CONSTANT WEIGHT METHOD
Definition – Moisture content of food sample is loss in mass on heating at 105±10 °C under specific operating conditions like humidity, pressure etc.

Principal – Food sample is dried in an oven at 105°C temperature till the constant weight is obtained from loss in weight. Then weight percent is calculated.
Apparatus – 1 ) food sample 2) crucible or petridish 3) desicator 4) weighing machine
Procedure –
- Weigh 5 gm sample into clean,dry weighed crucible or petridish.
- Place it in an hot air oven at 105°C for 5 hrs
- Cool in desicator and weigh
- Repeat the process of drying, cooling and weighing until consistent loss in weight
- Calculate % moisture by formula ( % Moisture = Loss in weight * 100 / weight of sample )
2. VACUUM DRYING METHOD
By drying under less pressure (25–100 mm Hg) we can obtain a more removal of water and volatile compounds without decomposition just within a 3–6 hrs drying time. The following are important points to be consider

- Temperature of drying depends on the food sample (such as 70◦C for fruits and other high-sugar products)
- If the product has a high concentration of volatile compounds, you should consider the use of a correction factor to measure the loss
- Evaporation is an endothermic (heat absorbing) process. Thus, a pronounced cooling is observed. Because of the cooling effect of evaporation, when several samples are placed in an oven of this type, you will note that the temperature will drop.
- Do not attempt to compensate for the cooling effect by increasing the temperature, otherwise samples during the last stages of drying will be overheated.
- The drying time is a function of the total moisture present, nature of the food, surface area per unit weight of sample.
3. KARL FISCHER TITRATION
The Karl Fischer titration is particularly used for food products that show uneven results when heated or submitted to a vacuum.
Used for – determination of water in many low moisture foods such as dried fruits & vegetables, candies, chocolates, roasted coffee, oils and fats, or any low-moisture food high in sugar or protein.
Procedure –
- Take Karl Fischer reagent (KFR) & add directly as the titrant if the moisture in the sample is accessible.
- However, if moisture in a solid sample is not accessible to the reagent, the moisture is extracted from the food with an appropriate solvent (e.g methanol). (Particle size affects efficiency of extraction directly)
- Then the methanol extract is titrated with KFR.
- Before the amount of water found in a food sample can be determined, a KFR water (moisture) equivalence (KFReq) must be determined.
- The KFReq can be established with pure water, a water-in-methanol standard, or sodium tartrate dihydrate.
The KFReq is calculated as follows using sodium tartrate dihydrate :
KFReq (mg H2O/ml) = 36 g H2O/mol Na2C4H4O6 · 2H2O × S × 1000 × 230.08 g/mol × A
- KFReq = Karl Fischer reagent moisture equivalence
- S = weight of sodium tartrate dihydrate (g)
- A = ml of KFR required for titration of sodium
Moisture content of the sample is determined as follows:
%Moisture = KFReq × Ks × S × 100
- KFReq = Karl Fischer reagent water (moisture)equivalence
- Ks = ml of KFR used to titrate sample
- S = weight of sample (mg)
The major difficulties and sources of error in the Karl Fischer titration methods are as follows:
- Incomplete moisture extraction
- Atmospheric moisture (External air must not be allowed to affect the reaction chamber)
- Interfere of certain food constituents. (Ascorbic acid is oxidized by KFR to dehydro-ascorbic acid to overestimate moisture content)
4.RAPID MOISTURE ANALYZER TECHNOLOGY
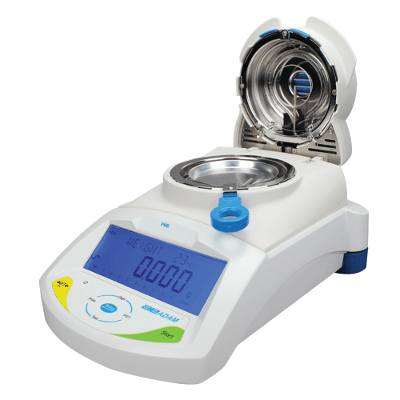


Rapid analyzers detect moisture levels from 50 ppm to 100%, using sample weights of 150 mg to 40 g sample.
- Using a digital balance, the test sample is placed on an aluminum pan or filter paper & the heat control program (with a heating range of 25–275◦C) heats the test sample to a constant temperature.
- As the moisture is removed from the sample, the instrument automatically weighs and calculates the percentage moisture
Advantages – This technology is utilized to cover a wide range of applications within the food industry and offers quick and accurate results within minutes. These analyzers are utilized for both production and laboratory use with results comparable to reference methods.
5. REFLUX DISTILLATION METHOD with Immiscible Solvent
Reflux distillation uses a solvent less dense than water (e.g toluene, with a boiling point of 110.6° C; or xylene, with a boiling range of 137–140°C & hexane is used depends on Nature of the food sample)

Procedure –
- Place sample in distillation flask and cover completely with solvent
- Fill the receiving tube (e.g., Bidwell-Sterling Trap) with solvent, by pouring it through the top of the condenser
- Bring to a boiling temperature & distill slowly at first, then at increased rate
- After the distillation has proceeded for approximately 1 hr, use an adapted buret brush to remove moisture droplets from the condenser and top part of the Bidwell-Sterling trap
- Slide the brush in a condenser to a point above the vapor condensing area
- Rinse the brush and wire with a small amount of toluene to remove adhering water drops
- If water has adhered (sticked) to the walls of the calibrated tube, invert the brush and use the straight wire to remove this water so it collects in the bottom of the tube
- Return the wire to a point above the condensation point, and rinse with another small amount of toluene
- After no more water has distilled from the sample, repeat the brush and wire method to remove adhering water droplets
- Rinse the brush and wire with toluene before removing from the condenser
- Allow the apparatus to cool to ambient temperatures before measuring the volume of water in the trap
- measure moisture by formula (Volume of water x 2 (for a 50 g sample) = % moisture)
Thank You !
Subscribe Us & get our latest content delivered to your inbox